Pipeline
Abon is currently seeking global partners for manufacturing and distribution for multiple proprietary technologies, as well as complex generics in the Long Acting Injectable space.
505(b)(2) Portfolio:
- Extended-release oral dosage form for poorly soluble amine drugs. Patent # US 9,011,912 B2
- Oral transmucosal dosage form offering superior patient compliance, safety and efficacy for the treatment of Hypogonadism. Patent # US 9,352,047 B2 & # US 10,821,118 B2
- Oral transmucosal dosage form offering superior patient compliance, safety and efficacy for Hormone Replacement Therapy (HRT). Patent # US 9,795,616 B2
- Long Acting Injection from Solution for psychotherapeutic drug. Patent Filed
- Long Acting Injection from Suspension for psychotherapeutic drug. Patent Filed
- Intravenous Delivery System for chemotherapy drugs. Patent Filed
- Improved delivery system for an inhibitor of glucosylceramide synthase to overcome metabolic variability. Patent Filed
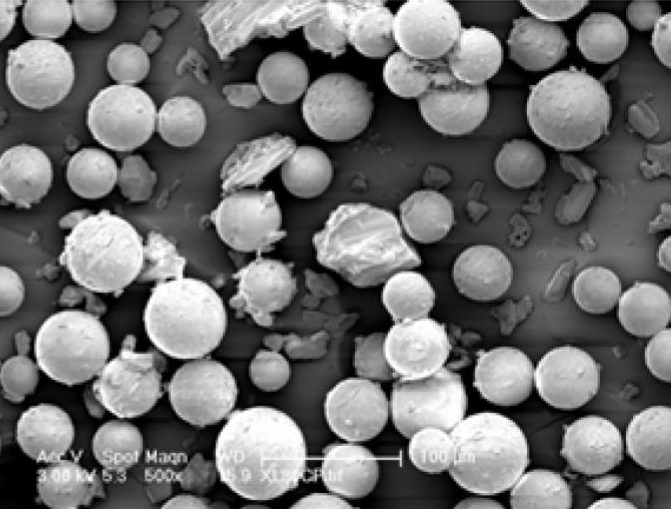
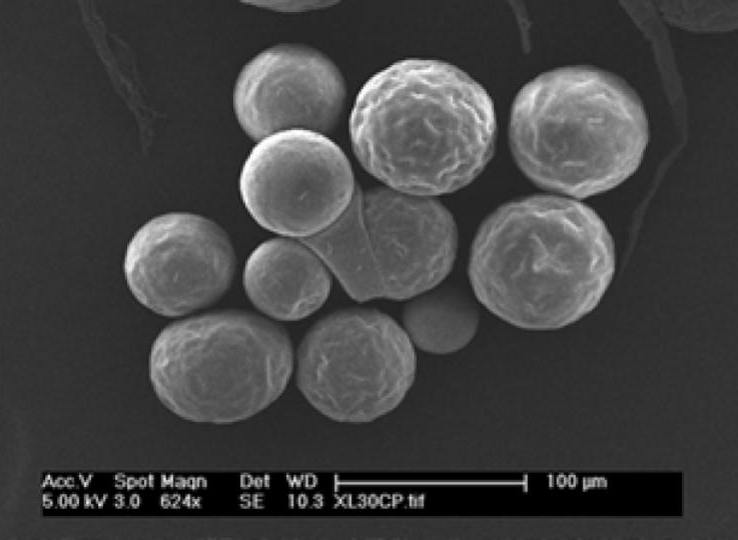
Long-Acting Injectable Generics:
- Polypeptide for cancer treatment
- Biodegradable microsphere for a psychotherapeutic drug
- Biodegradable microsphere for acromegaly and certain cancers
- Microparticle Suspension for a psychotherapeutic drug

© 2021 Abon Pharmaceuticals, LLC
